Solar Cooling | Seminar Report for B.Tech Mechanical Students
Solar Cooling | Seminar Report for B.Tech Mechanical Students
Solar cooling refers to any cooling system that uses solar power. This can be done through passive solar, solar thermal energy conversion and photovoltaic conversion (sun to electricity).
The U.S. Energy Independence and Security Act of 2007 created 2008 through 2012 funding for a new solar air conditioning research and development program, which should develop and demonstrate multiple new technology innovations and mass production economies of scale. Solar air conditioning will play an increasing role in zero energy and energy-plus buildings design.
Solar energy, radiant light and heat from the sun, has been harnessed by humans since ancient times using a range of ever-evolving technologies. Solar radiation, along with secondary solar-powered resources such as wind and wave power, hydroelectricity and biomass, account for most of the available renewable energy on earth. Only a minuscule fraction of the available solar energy is used.
Solar powered electrical generation relies on heat engines and photovoltaic. Solar energy’s uses are limited only by human ingenuity. A partial list of solar applications includes space heating and cooling through solar architecture, potable water via distillation and disinfection, day lighting, solar hot water, solar cooking, and high temperature process heat for industrial purposes. To harvest the solar energy, the most common way is to use solar panels.
Solar technologies are broadly characterized as either passive solar or active solar depending on the way they capture, convert and distribute solar energy. Active solar techniques include the use of photovoltaic panels and solar thermal collectors to harness the energy. Passive solar techniques include orienting a building to the Sun, selecting materials with favorable thermal mass or light dispersing properties, and designing spaces that naturally circulate air.
Introduction
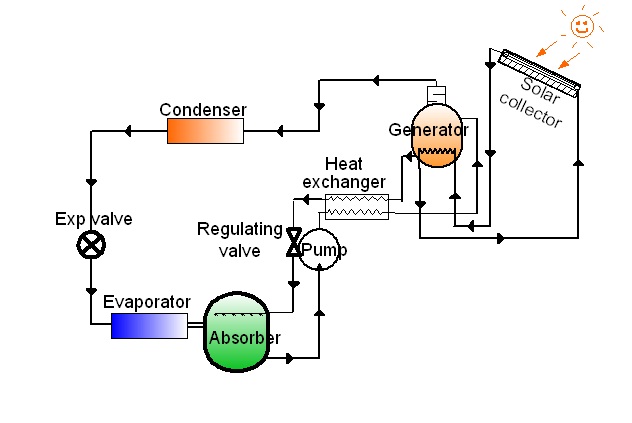
The core idea is to use the solar energy directly to produce chilled water. The high temperature required by absorption chillers is provided by solar troughs. The system doesn’t require “strategic” materials (like in PV systems) and has peak production in the moment of peak demand.
Why Solar Cooling?
Dramatic increase of air conditioning since the early 80ies
• Cost of energy.
• Issues related to environmental pollution.
-Due to energy production.
-Due to the use of CFC’s and HCFC’s.
• Matches demand with source availability.
• Crucial for improving life standards in developing countries.
Thermal Solar Cooling Techniques
Absorption cooling techniques:
Energy is transferred through phase-change processes.
Adsorption cooling techniques
Energy is transferred through phase-change processes.
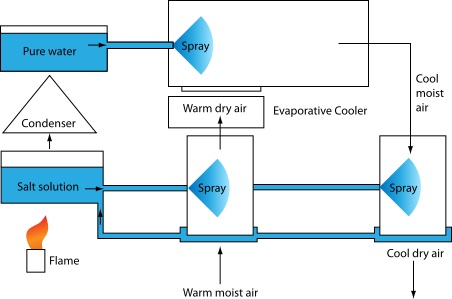
Desiccant Cooling
Energy is transferred through latent heat processes.
“The cooling capacity is based on the physical properties of the cooling fluid that will change phases. At different temperatures, depending on its pressure.”
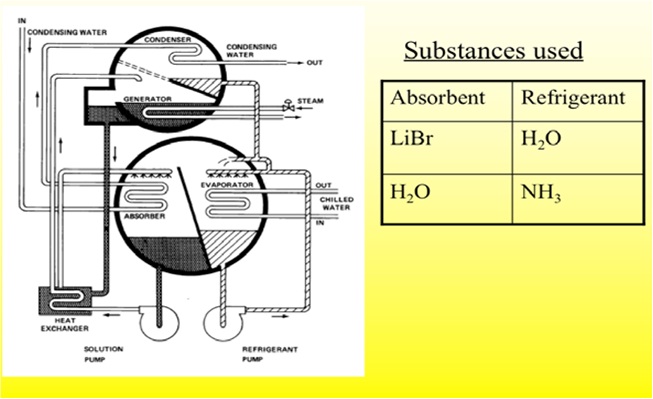
Absorption Cooling
Heat Driven Systems
Absorption Refrigerator-
The absorption refrigerator is a refrigerator that uses a heat source (e.g., solar, kerosene-fueled flame) to provide the energy needed to drive the cooling system. Absorption refrigerators are a popular alternative to regular compressor refrigerators where electricity is unreliable, costly, or unavailable, where noise from the compressor is problematic, or where surplus heat is available (e.g., from turbine exhausts or industrial processes). Absorption refrigerators powered by heat from the combustion of liquefied petroleum gas are often used for food storage in recreational vehicles.
Both absorption and compressor refrigerators use a refrigerant with a very low (less than 0 °F/−18 °C) boiling point. In both types, when this refrigerant evaporates or boils, it takes some heat away with it, providing the cooling effect. The main difference between the two types is the way the refrigerant is changed from a gas back into a liquid so that the cycle can repeat. A compressor refrigerator uses an electrically-powered compressor to increase the pressure on the gas, and then condenses the hot high pressure gas back to a liquid by heat exchange with a coolant (usually air). Once the high pressure gas has cooled, it passes through a pressure release valve which drops the refrigerant temperature to below freezing. An absorption refrigerator changes the gas back into a liquid using a different method that needs only heat, and has no moving parts. The other difference between the two types is the refrigerant used. Compressor refrigerators typically use an HCFC, while absorption refrigerators typically use ammonia
Adsorption Refrigeration
Adsorption refrigeration and heat pump cycles rely on the adsorption of a refrigerant gas into an adsorbent at low pressure and subsequent desorption by heating. The adsorbent acts as a “chemical compressor” driven by heat and is, from this point of view, the “pump” of the system. It consists of a solar collector, a condenser or heat-exchanger and an evaporator that is placed in a refrigerator box. The inside of the collector is lined with an adsorption bed packed with activated carbon adsorbed with methanol. The refrigerator box is insulated filled with water. The activated carbon can adsorb a large amount of methanol vapours in ambient temperature and desorb it at a higher temperature (around 100 degrees Celsius). During the daytime, the sunshine irradiates the collector, so the collector is heated up and the methanol is desorbed from the activated carbon. In desorption, the liquid methanol adsorbed in the charcoal heats up and vaporizes. The methanol vapour condenses and is stored in the evaporator.
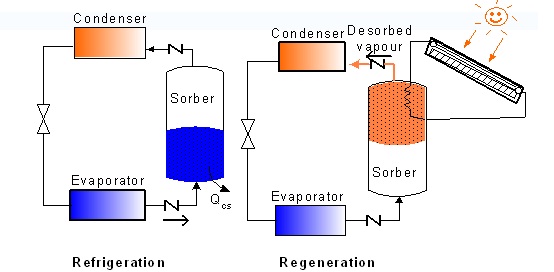
Helium gas can also be ‘pumped’ by thermally cycling activated carbon ‘sorption pumps’ between 4 kelvins and higher temperatures. An example of this is to provide the cooling power for the Oxford Instruments AST series dilution refrigerators. 3He vapour is pumped from the surface of the dilute phase of a mixture of liquid 4He and its isotope 3He. The 3He is adsorbed onto the surfaces of the carbon at low temperature (typically <4K), the regeneration of the pump between 20 and 40 K returns the 3He to the concentrated phase of the liquid mixture. Cooling occurs at the interface between the two liquid phases as 3He ‘evaporates’ across the phase boundary. If more than one pump is present in the system a continuous flow of gas and hence constant cooling power can be obtained, by having one sorption pump regenerating while the other is pumping. Systems such as this allow temperatures as low as 10 mK (0.01 kelvin) to be obtained with very few moving parts.
Desiccant system
A desiccant is a hygroscopic substance that induces or sustains a state of dryness (desiccation) in its local vicinity in a moderately well-sealed container.
Commonly encountered pre-packaged desiccants are solids, and work through absorption or adsorption of water, or a combination of the two. Desiccants for specialized purposes may be in forms other than solid, and may work through other principles, such as chemical bonding of water molecules.
Pre-packaged desiccant is most commonly used to remove excessive humidity that would normally degrade or even destroy products sensitive to moisture. Drierite, Silica gel, calcium sulfate, calcium chloride, montmorillonite clay, and molecular sieves are commonly used as desiccants.
Rice is a common “low-tech” alternative frequently used, for example, in salt-shakers to maintain granularity of table-salt for effective pouring or shaking. Rice, however, is not a good general purpose desiccant since, unless immersed in an organism-hostile environment like pure salt, over time may be eaten by creatures that might in turn contaminate the product that is being preserved. Salt itself is another effective desiccant, used for millennia in preparation of dried food and also to mummify corpses.
Solar Cooling Path and Local Conditions
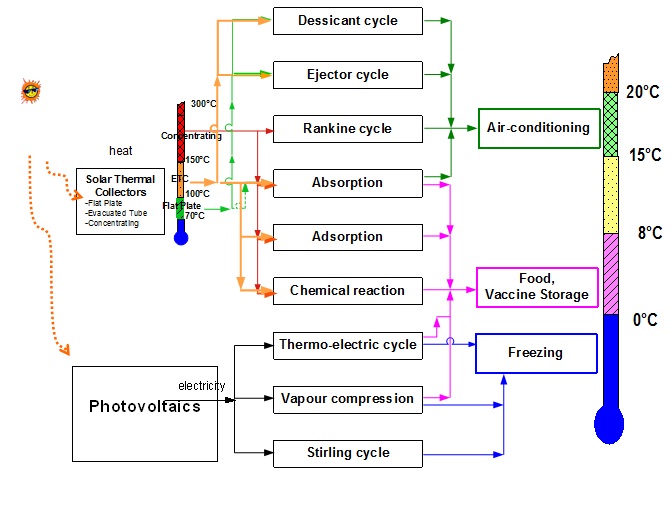
Refrigerants
A vapor-compression chiller uses a refrigerant internally as its working fluid. Many refrigerants options are available; when selecting a chiller, the application cooling temperature requirements and refrigerant’s cooling characteristics need to be matched. Important parameters to consider are the operating temperatures and pressures.
There are several environmental factors that concern refrigerants, and also affect the future availability for chiller applications. This is a key consideration in intermittent applications where a large chiller may last for 25 years or more. Ozone depletion potential (ODP) and global warming potential (GWP) of the refrigerant need to be considered. ODP and GWP data for some of the more common vapor-compression refrigerants:
“Freon” is a trade name for a family of haloalkane refrigerants manufactured by DuPont and other companies. These refrigerants were commonly used due to their superior stability and safety properties: they were not flammable nor obviously toxic as were the fluids they replaced, such as sulfur dioxide. Unfortunately, these chlorine-bearing refrigerants reach the upper atmosphere when they escape. In the stratosphere, CFCs break up due to UV-radiation, releasing their chlorine atoms. These chlorine atoms act as catalysts in the breakdown of ozone, which does severe damage to the ozone layer that shields the Earth’s surface from the Sun’s strong UV radiation. The chlorine will remain active as a catalyst until and unless it binds with another particle, forming a stable molecule. CFC refrigerants in common but receding usage include R-11 and R-12. Newer refrigerants that have reduced ozone depletion effect include HCFCs (R-22, used in most homes today) and HFCs (R-134a, used in most cars) have replaced most CFC use. HCFCs in turn are being phased out under the Montreal Protocol and replaced by hydrofluorocarbons (HFCs), such as R-410A, which lack chlorine. However, CFCs, HCFCs, and HFCs all have large global warming potential.
Newer refrigerants are currently the subject of research, such as supercritical carbon dioxide, known as R-744. These have similar efficiencies compared to existing CFC and HFC based compounds, and have many orders of magnitude lower global warming potential.
CONCLUSIONS
The thermodynamics of the vapor compression cycle can be analyzed on a temperature versus entropy diagram. At point 1 in the diagram, the circulating refrigerant enters the compressor as a saturated vapor. From point 1 to point 2, the vapor is isentropically compressed (i.e., compressed at constant entropy) and exits the compressor as a superheated vapor.
From point 2 to point 3, the superheated vapor travels through part of the condenser which removes the superheat by cooling the vapor. Between point 3 and point 4, the vapor travels through the remainder of the condenser and is condensed into a saturated liquid. The condensation process occurs at essentially constant pressure.
Between points 4 and 5, the saturated liquid refrigerant passes through the expansion valve and undergoes an abrupt decrease of pressure. That process results in the adiabatic flash evaporation and auto-refrigeration of a portion of the liquid (typically, less than half of the liquid flashes). The adiabatic flash evaporation process is isenthalpic
REFERENCES
• Solar assisted air-conditioning of buildings –an overview
Hans-Martin Henning and Edo Wiemken
• Desiccant cooling technology powered by solar thermal air collector systems
Ursula Eicker, Martin Huber, Uwe Schurger, Jurgen Schumacher, Andreas Trinkle
• Prototype for a novel solar powered ejector air-conditioning system in Mazunte, Mexico
J.L. Wolpert, S.B. Riffat and S. Redshaw